Iron in the presence of moisture combines with the oxygen from the air to form a brown-coloured chemical substance called rust (iron oxide). There is no physical process to get back iron from the rust (iron oxide).
Rusting is a costly nuisance . Methods of slowing down the process can save a lot of money . Many metals become corroded by exposure to the air. The corrosion of iron and steel is called rusting. The most common example of corrosion is that corrosion comes off from the metal surface, rusting of iron causing iron to change into rust – an orange – red powdery a fresh metal surface is exposed and the process of corrosion continues.

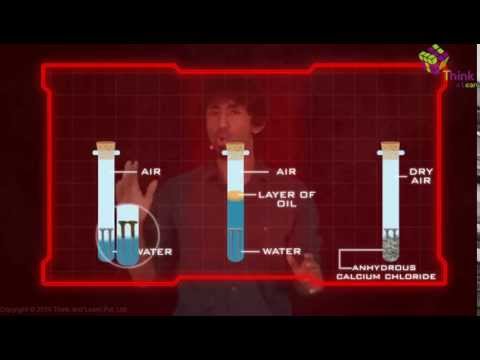
The necessary condition for rusting are
Both oxygen and water (or moisture) must be present for the rusting of iron to occur. So two conditions are necessary for the rusting of iron to take place.
Iron rusts when placed in damp air, or when placed in water. Now, damp air also contains water vapour. Thus damp air provides both the things, oxygen and water required for the rusting of iron to occur. Again, ordinary water always has some dissolved oxygen in it. So ordinary water also supplies both the things, oxygen and water, needed for the rusting of iron.
A reddish-brown deposit called rust, forms over a piece of iron when it is exposed to moist air for some time. Rust is hydrated iron (III) oxide (Fe2O3.xH2O). The rusting of iron is a chemical change because we cannot get back pure iron from rusted iron by reversing the conditions.
Rusting takes place when an iron object comes in contact with air and water. So if air and water are prevented from coming in contact with iron objects, the rusting will not take place. Thus most of the methods of preventing rusting of iron involve coating the iron objects with “something” to keep out air and water. Some of the methods of preventing rusting of iron are given below.
It can be prevented from rusting by anything as easy as painting the surface of the iron. Painting creates a barrier over the surface of iron, much as in galvanization. A boundary between the iron and its surrounding atmosphere is formed by the presence of the dye. However, the downside of painting iron is that it does not last as long as other treatments of the surface.
Oil not only lubricates and makes it easier to move metal pieces with less friction, but oil also provides a protective shield against corrosion. The idea here is fairly simple; moisture will not interfere with the iron in the metal with a coating of oil and create corrosion. The use of oil or grease substantially decreases the risk of developing iron corrosion. Basically, just like ink does, grease or oil forms a protective coating over iron and thereby avoids rust forming.
Galvanising is a preventive tool for corrosion. A thin layer of zinc coated the iron or steel object. This prohibits oxygen and water from accessing the underlying metal-but zinc also serves as a sacrificial metal. Zinc is more reactive than iron, but in preference to the iron object, it oxidises. Galvanised metal, such as storage sheds, chain-link fencing, and metal on boats, is most commonly used on outdoor objects or anything likely to be in contact with moisture or water.
Chromium, because of its properties, is used for electroplating. After electroplating, it is glossy, which makes the material appealing. It is immune to corrosion and scrape. Thus, it prevents the item from scratched and corrosion. In the sense that chromium is the only elemental solid that exhibits antiferromagnetic order at room temperature (and below), chromium has unique magnetic properties. Its magnetic ordering shifts to paramagnetic, over 38 ° C.
The generic name of a very generic compound, iron oxide, is rust. Iron oxide, the Fe2O3 chemical, is abundant because iron is so easily mixed with oxygen — so readily, in fact, that pure iron is rarely present in nature.
A red or orange layer, composed primarily of ferric hydroxide and ferric oxide created by oxidation, which forms on the surface of iron when exposed to air and moisture. Any oxidation-caused film or coating on aluminium.
Rusting is a reaction of oxidation. The iron reacts to hydrated iron(III) oxide, which we see as rust, with water and oxygen to form. When they come into contact with water and oxygen, iron and steel rust-both are needed for rusting to happen.
However, the key distinction between corrosion and rust is that corrosion happens as a result of chemical effects and affects multiple materials, whereas rusting is only accelerated by some chemicals and typically affects iron substances.
By splitting the oxygen atom, water makes iron react with oxygen. Iron loses electrons and oxygen gains electrons during the early stages of rusting. Ferrous and ferric ions then react to ferrous hydroxide, ferric hydroxide and hydrogen with water to form.